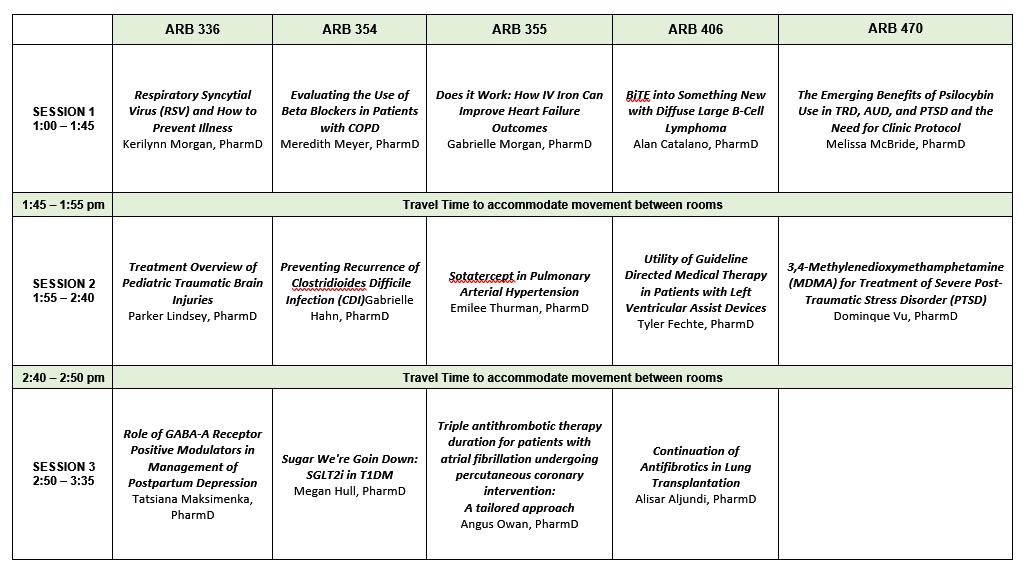

- ARB 336: Click here to join via Microsoft Teams
- ARB 354: Click here to join via Microsoft Teams
- ARB 355: Click here to join via Microsoft Teams
- ARB 406: Click here to join via Microsoft Teams
- ARB 470: Click here to join via Microsoft Teams
ATTENDANCE & EVALUATION: Attendance will be collected for both in-person and live participants. To receive CPE credit, pharmacists must complete the evaluation for each activity attended (maximum 2.25 contact hours for 3 sessions). The evaluation link will close on November 15; CPE credit may no longer be claimed after that time. CPE credit will not be corrected or awarded if more than 60 days have passed from the event.
SPECIAL ACCOMMODATIONS
Attendees of all abilities are welcome to participate. If you will require accommodations, please notify ce@uhsp.edu
Date: Nov 8, 2023 01:00 PM - 03:35 PM
Fee
CE Hours
Activity Type
- Knowledge
Objectives
- Describe the pathophysiology and treatment of interstitial lung disease
- Summarize literature on antifibrotic use in the peri-operative lung transplant period
- Evaluate considerations for discontinuation of antifibrotics prior to lung transplant
Speaker(s)/Author(s)
|
Alisar Aljundi, PharmD |
Activity Number
0033-0000-23-019-L01-PCE Hours
Objectives
- Explain current treatment landscape of DLBCL
- Describe treatment outcomes of relapsed/refractory DLBCL
- Identify differences between current FDA approved bispecific T-cell engagers for DLBCL
Speaker(s)/Author(s)
|
Alan Catalano, PharmD |
Activity Number
0033-0000-23-022-L01-PCE Hours
Objectives
- Compare and contrast mechanisms of action for anticoagulants and antiplatelets.
- Identify the appropriate antithrombotic treatment regimen and duration for a patient with atrial fibrillation undergoing percutaneous coronary intervention.
Speaker(s)/Author(s)
|
Angus Owan, PharmD |
Activity Number
0033-0000-23-020-L01-PCE Hours
Objectives
- Discuss the prevalence and symptoms of Post-Traumatic Stress Disorder
- Identify the current recommendations for treatment of PTSD
- Describe 3,4 methylenedioxymethamphetamine’s place in therapy for management of PTSD
Speaker(s)/Author(s)
|
Dominique Vu, PharmD |
Activity Number
0033-0000-23-021-L01-PCE Hours
Objectives
- Identify the primary physiologic pathways involved in the pathophysiology of pulmonary arterial hypertension (PAH)
- Describe the mechanism of action of sotatercept in the treatment of PAH
- Explain the role of sotatercept among current therapies for PAH
Speaker(s)/Author(s)
|
Emilee Thurman, PharmD |
Activity Number
0033-0000-23-023-L01-PCE Hours
will be discussed as they apply to CDI recurrence. The focus of the presentation will be to explore the role of pharmacotherapy in recurrence prevention, comparing current guideline recommendations and newly approved agents, Rebyota and Vowst.
Objectives
- Identify the risk factors contributing to recurring CDI.
- Compare and contrast prevention strategies - Bezlotoxumab, Rebyota, and Vowst
- Determine most appropriate preventative strategy given a patient case.?
Speaker(s)/Author(s)
|
Gabrielle Hahn, PharmD |
Activity Number
0033-0000-23-024-L01-PCE Hours
Objectives
- Describe the difference in outcomes in congestive heart failure (CHF) patients with iron deficiency compared to CHF patients without iron deficiency.
- Determine if intravenous (IV) iron would benefit a patient exhibiting iron deficiency.
- Identify the best iron replacement product for a patient’s condition.
Activity Number
0033-0000-23-025-L01-PCE Hours
Objectives
- Describe the prevalence of RSV illness and the risk factors for severe RSV illness.
- Discuss the literature on the different available preventative therapies.
- Explain the Advisory Committee on Immunization Practices (ACIP) and the American College of Obstetricians and Gynecologist (ACOG) RSV vaccine recommendations.
Speaker(s)/Author(s)
|
Kerilynn Morgan, PharmD |
Activity Number
0033-0000-23-026-L01-PCE Hours
Objectives
- Summarize the mechanism and utility of SGLT2 inhibitors.
- Describe type 1 diabetes epidemiology and pathophysiology.
- Identify key outcomes and conclusions of trials studying SGLT2-i utility in T1DM.
- Recognize the appropriate place in therapy for SGLTi in treating T1DM.
Speaker(s)/Author(s)
|
Megan Hull, PharmD |
Activity Number
0033-0000-23-027-L01-PCE Hours
Objectives
- Explain how treatment with psilocybin may be advantageous for TRD, AUD, and PTSD
- Define the current laws in place in Missouri for the use of psilocybin
- Distinguish how pharmacists may play a role in treatment with psilocybin in the future
Speaker(s)/Author(s)
|
Melissa McBride, PharmD |
Activity Number
0033-0000-23-032-L01-PCE Hours
Objectives
- From a pathophysiologic standpoint, describe the mechanism of action of beta blockers and why they are underprescribed in patients with chronic obstructive pulmonary disease (COPD)
- Evaluate the risks and benefits of beta blocker therapy in a patient with COPD.
- Compare and contrast the data regarding cardioselective and noncardioselective beta blocker use in patients with COPD to make an informed and patient-specific recommendation
Speaker(s)/Author(s)
|
Meredith Meyer, PharmD |
Activity Number
0033-0000-23-028-L01-PCE Hours
Objectives
- Describe the pathophysiology and common etiologies of traumatic brain injury (TBI)?
- Identify the components commonly included in treatment plans for pediatric TBIs
- Implement appropriate pharmacotherapy choices based on a patient’s clinical presentation
Speaker(s)/Author(s)
|
Parker Lindsey, PharmD |
Activity Number
0033-0000-23-030-L01-PCE Hours
Objectives
- Discuss risk factors, etiology, and pathophysiology of postpartum depression (PPD)
- Discuss guidelines recommendations for PPD treatment
- Discuss clinical trials representing effectiveness and safety associated with the use of GABA- A Receptor Positive Modulators for PPD treatment
Speaker(s)/Author(s)
|
Tatsiana Maksimenka, PharmD |
Activity Number
0033-0000-23-029-L01-PCE Hours
Objectives
- Compare and contrast GDMT medications and use in patients with LVADs.
- Describe potential risks and benefits of LVAD for bridging to transplant, destination therapy, or bridge to recovery
- Recommend appropriate GDMT agents for patients with LVADs
Speaker(s)/Author(s)
|
Tyler Fechte, PharmD |
